
STERIGENE, a major supplier in the field of ultra-cleanliness and an expert in project management, has joined the SYNEXIN group since the beginning of 2022. Through the fusion of process knowledge and experience in project management for the pharmaceutical industry of its entities, SYNEXIN is able to support you in carrying out your project.
SYNEXIN brings together several entities competent in clean and sterile processes, in aeraulic expertise, in the design and layout of critical areas as well as in clean room consumables. It is not just a matter of offering the equipment, furniture or consumables you need for your project, but of supporting you during its implementation with overall management: from implementation to finalization through qualification. of your equipment or your critical environments via dedicated technical monitoring.
SYNEXIN is a company that brings together several entities, all specialized in different professions linked to critical environments.
Need some information ?

The single point of contact for the engineering of your clean and sterile processes: process equipment, technical services, consumables and personnel equipment.

The specialist in the field of controlled environments: equipment and components for aeraulic systems, qualification of critical environments.

The expert in the integration and manufacturing of tailor-made furniture for clean rooms: viewing tables, passage benches, furniture, consumable dispensers and changing rooms.
With this knowledge and skills, SYNEXIN is able to propose and establish a validation strategy for your project based on process risk analyzes as well as the design qualifications of your projects.
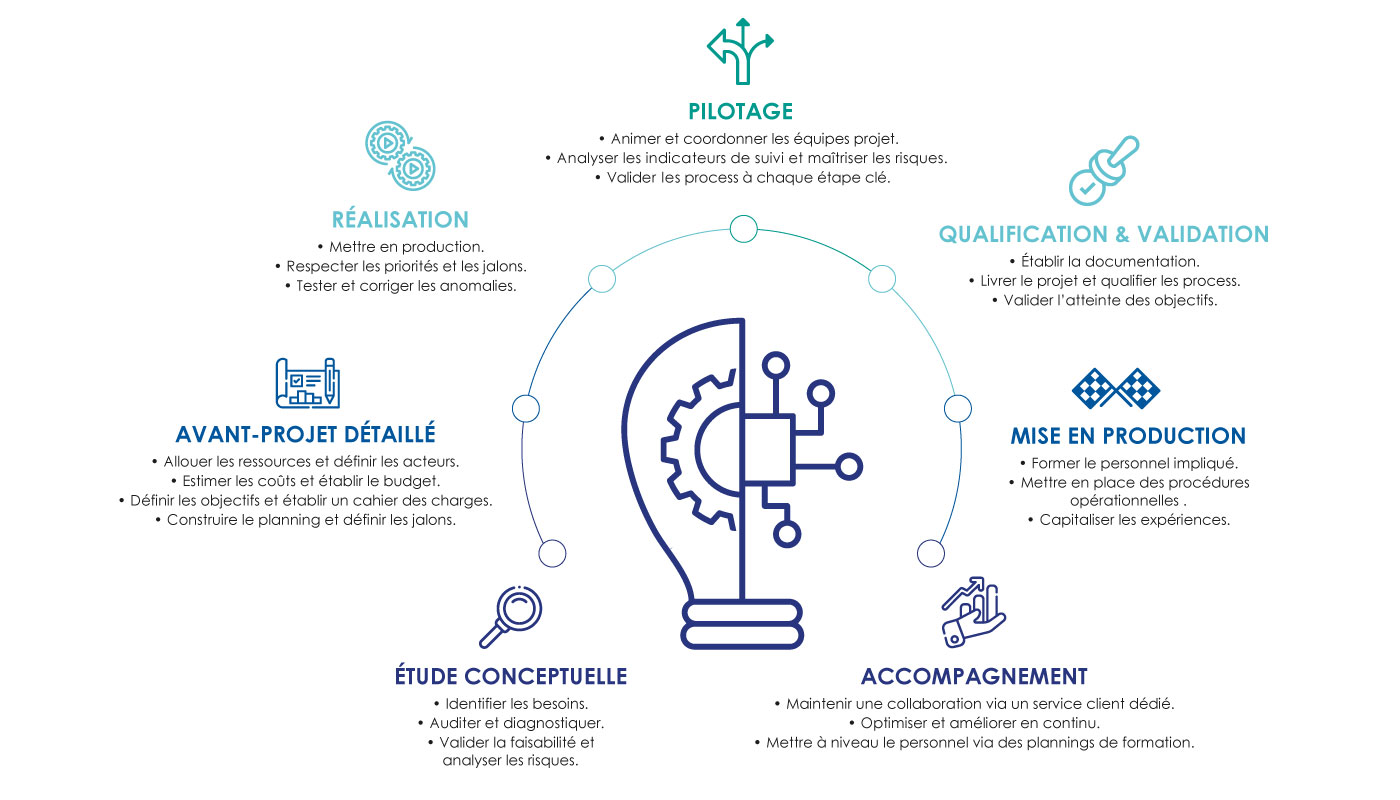
OUR METHOD
VALIDATION MASTER PLAN
The validation master plan identifies the strategies and tools that can be used to carry out and control the commissioning and qualification activities necessary to ensure the transfer of all installations to operators.
According to Annex 15 of the GMP, the validation master plan or equivalent document must define the qualification / validation system and include or reference at least information on the following elements:
- The qualification and validation policy;
- Reference standards and regulations;
- The organizational structure including roles and responsibilities for qualification and validation activities;
- Summary of the site’s installations, equipment, systems and processes and their qualification and validation status;
- Control of changes and management of deviations applied to qualification and validation;
- Recommendations for determining acceptance criteria;
- References to existing documents;
- The qualification and validation strategy, including re-qualification, if applicable.
It also allows you to:
- Specify the limits or scope of action between the customer and supplier
- Specify a macro schedule for the validation strategy
- Control the transfer of installations (transfer from suppliers to the customer and transfer of the project to operators)
RISK ANALYSIS
Process risk analysis is an essential step in project management. It is :
- Carry out a risk inventory for all stakeholders (maintenance, operators, metrology)
- Value risks with a risk rating according to severity calculated from occurrence, frequency and detectability
- Define appropriate solutions and tests according to the risk rating in order to reduce their occurrence.
All these steps aim to identify the critical elements which will necessarily be integrated into the qualifications. We therefore offer you a file including:
- A summary of each function of your system
- A summary of the risks associated with each function
- Identification of the causes and consequences of each risk as well as their criticality
- Possible means of controlling risks
- Developing the validation strategy via defining validation tests
This file is established according to the regulatory standards below.
| REFERENCE | TITLE/SOURCE |
| GMP revised on 11/26/2020 | Good Manufacturing Practices |
| EU GMP – Annex 1 08/22/2022 | Manufacturing of sterile medicines |
| GMP – Annex 11 | Computerized systems |
| GMP – Annex 15 | Qualification and Validation |
| ISO 9001 rev 2015 | Standard for Quality Management Systems |
| ICH Q9 | Harmonized Tripartite Guideline “Quality Risk management” |
| ISPE Baseline guide Vol5 | ISPE baseline Pharmaceutical Engineering Guide for New and Renovated Facilities, Vol5 “Commissioning and Qualification” |
| ISPE Baseline Guide Vol12 (Gamp 5) | Science and Risk Based Approach for the Delivery of Facilities, Systems and Equipment |
DESIGN QUALIFICATION
The design qualification is the document in which the conformity of the design with the required standards must be demonstrated and documented. The requirements formulated in the user specifications must also be verified during design qualification.
We offer you a document listing point by point the requirements of your specifications as well as those of the GMP relating to your system, in which each conformity is notified then justified by the indication of the supplier document.
This document therefore makes it possible to highlight points of attention, risk, or deviations upstream of implementation and to trigger appropriate corrective actions.


