The manufacture of sterile products requires a rigorous, multi-dimensional contamination control strategy (CCS) : design of premises, qualification of aeration systems, viable and non-viable monitoring, and documented disinfection programmes.
These principles are at the heart of Annex 1 of Good Manufacturing Practice (GMP), which sets out the regulatory expectations for preventing microbial contamination and ensuring the safety of sterile medicines.
In recent years, Far-UVC technology has attracted growing interest as a complementary means of continuously reducing the microbial load in occupied environments : experimental studies show that this range of wavelengths effectively inactivates viruses and bacteria in suspension and on surfaces, while presenting, according to published data, a reduced risk of penetration into human tissue compared with conventional UVC.
These characteristics make Far-UVC technology an attractive option for reinforcing control barriers in pharmaceutical environments.
Need some information ?
FAR-UVC Technology
The atmosphere absorbs all light below ~300 nm. Hence UVC only exist from artificial sources, i.e., lamps, LED’s and lasers.
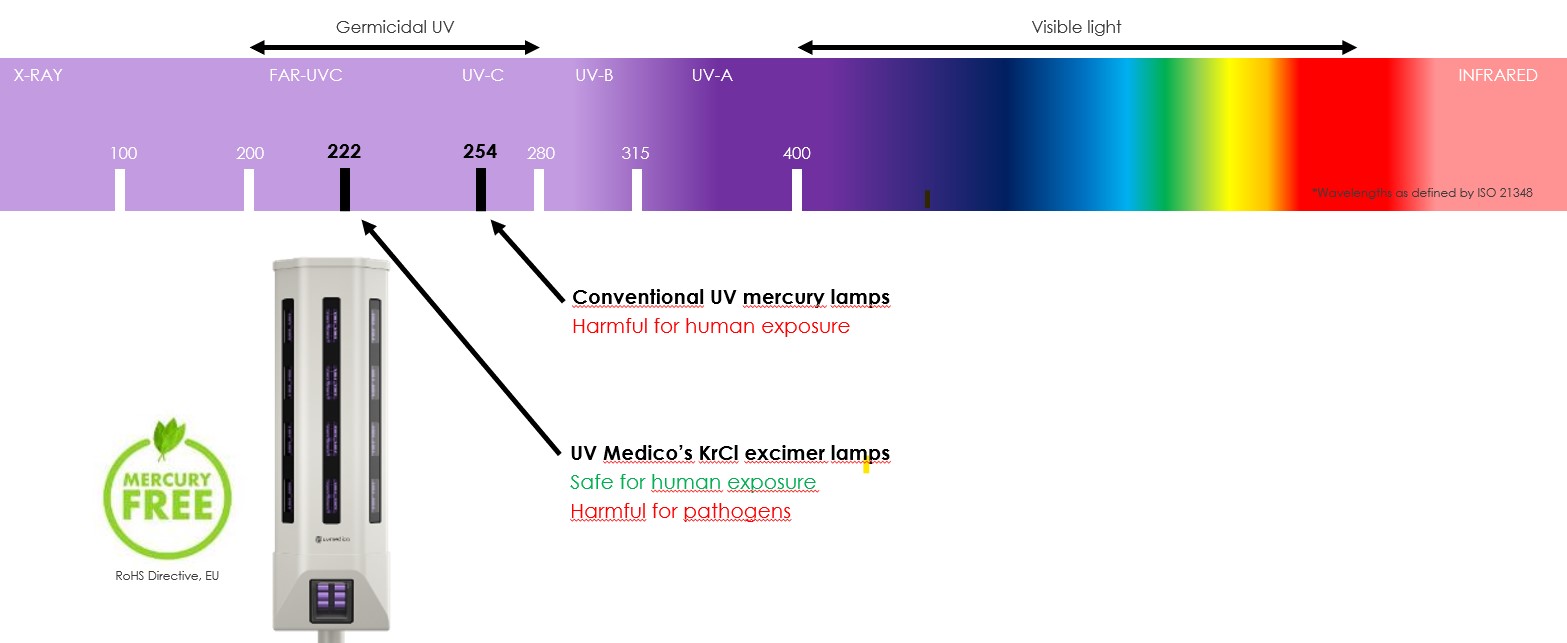
FAR-UVC security
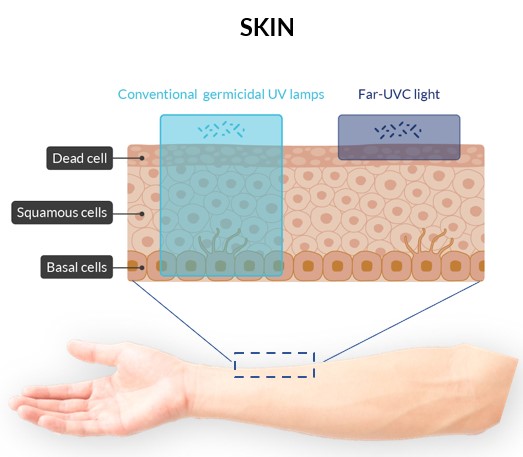
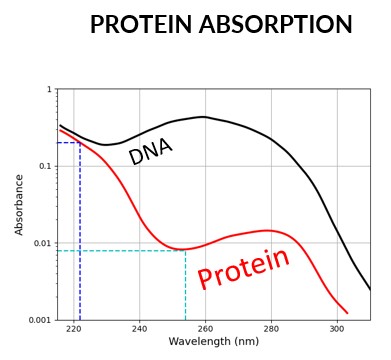
The top layer of skin (stratum corneum) is dense in proteins.
Proteins at 254 nm: low absorption
-> penetration through skin
Proteins at 222 nm: high absorption
-> negligible penetration through skin
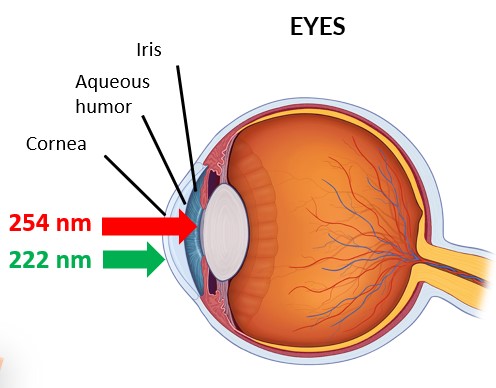
Transmission of 222 nm through the cornea :
-> negligible
The advantages of FAR-UVC technology


Safe for people
- Far-UVC can be used in occupied rooms due to its low penetration into eyes and skin
Effective, continuous decontamination
- Improves health, safety, and productivity
- Reduces spread of infectious diseases
- Increases hygiene standards
- Continuously reduces bioburden
Industry leadership
- Global footprint with multiple reference sites worldwide
- Installed in leading hospitals across Europe, the Middle East, and in major multinational pharmaceutical companies
- Proven performance in high-traffic public environments
Efficiency & Standards
Far UV-C Efficacy Scientifically Proven
Far UV-C at 222nm inactivates a wide spectrum of microbial pathogens.
Being safe for humans, its continuous use in occupied spaces substantially reducing the presence of fungal hyphae and spores in hospitals, thus reducing the risk of severe fungal infections.
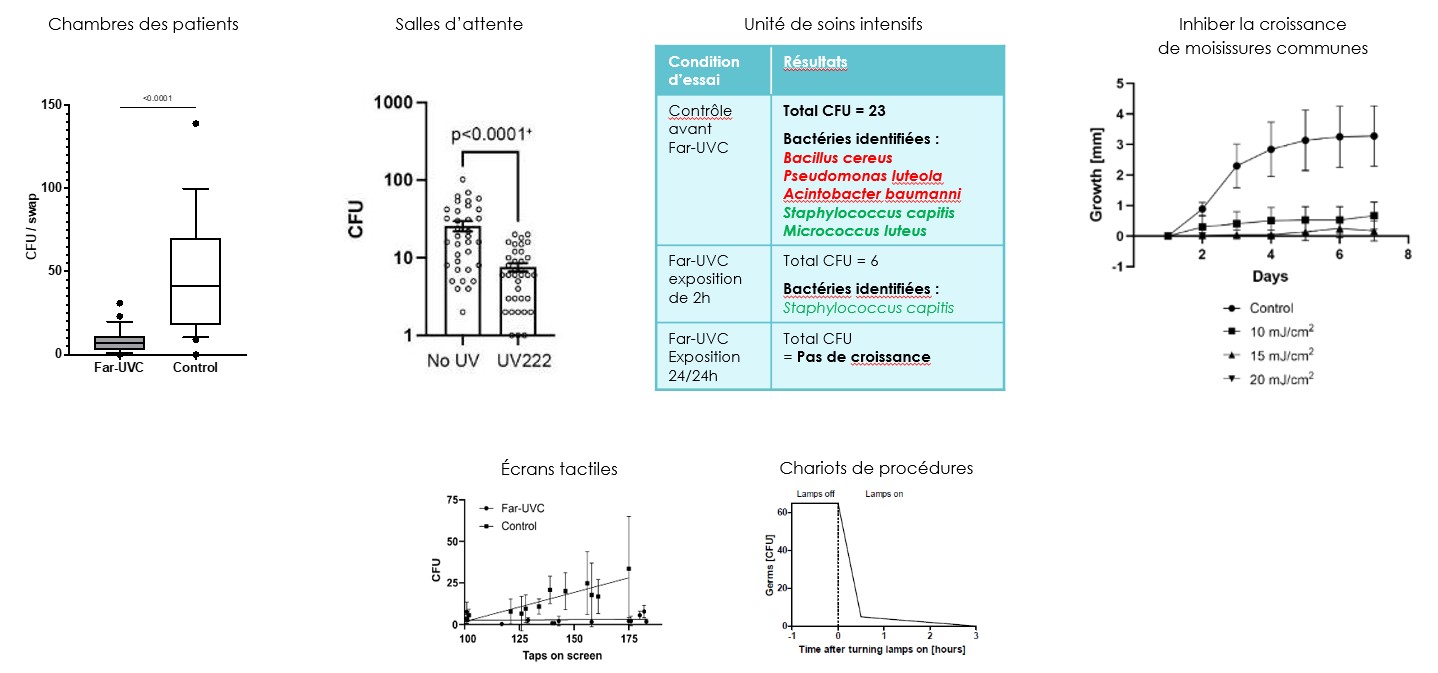
Elimination of bacteria in hospital settings
FAR-UVC technology helps comply with GMP Annex 1
Annex 1 of the European Union’s Good Manufacturing Practices (GMP) requires manufacturers of sterile medicinal products to implement a Contamination Control Strategy (CCS) with measures to minimise the risk of microbial, endotoxin/pyrogen and particulate contamination.
FAR-UVC technology can significantly reduce the risk of contamination, helping to comply with Annex 1 and reduce deviations.
FAR-UVC technology complies with :
International standards :
ISO 15858 – UV-C devices – Safety information – permissible human exposure.
IEC 62471 – Photobiological safety of lamps and lamp systems.
IEC PAS 63313 ED1 – Position statement on germicidal UV-C irradiation – UV-C safety guidelines (see GLA).
International guidelines:
ACGIH® – TVL 2022 (threshold values) & BEI (Biological Exposure Indices) for chemical substances and physical agents.
Integration into your Contamination Control Strategy (CCS)
Our UV222 solutions from our partner UV Medico help you solve the problems of contamination control strategies in cleanrooms:




> Decontaminate personnel or incoming materials
Find out more about the UV222 Stand, the UV222 Step-on and the Material SAS
> Prevent contamination in aseptic filling lines
Find out more about Linear UV222
> Continuously reduce the biological load in cleanroom work areas
Find out more about Spot UV222
> Minimise contamination in high-traffic areas
Find out more about the Vertex UV222

