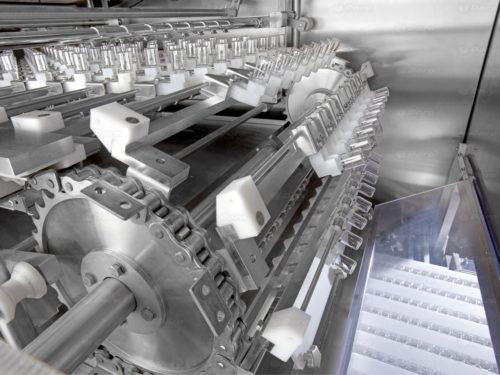
Automatic WM washing units
Washing




DARA WM washing tunnel enables the bottles, vials, syringes and cartridges washing, rinsing and drying for the pharmaceutical, cosmetic, biotechnological industries.
The DARA PHARMA washing tunnel enables the bottles, vials, syringes and cartridges washing, rinsing and drying for the pharmaceutical, cosmetic, biotechnological industries.
DARA PHARMA WM washing units are characterized by their flexibility and high performance with an output up to 36,000 uph.
The design has been made in accordance with the cGMP regulations.
DARA PHARMA WM washing units are characterized by their flexibility and high performance with an output up to 36,000 uph.
The design has been made in accordance with the cGMP regulations.
APPLICATIONS
- Pharmaceutical Industry
- Cosmetic Industry
- Biotechnology Industry
- Chemical Production Industry
- R&D Laboratory
- Analytic control Laboratory
- Food-processing Industry
PROCESS
Washing
CARACTÉRISTIQUES TECHNIQUES
- Contact parts AISI316L, frame and facades AISI304, tempered glass
- FDA compliant plastics (Teflon, Delrin), sanitary pump, hose clamp
- Filtration on washing fluid at 2.5 µm then 0.45 µm, sterile compressed air 0.2 µm
- Class C-D installation
CHARGES
Caps
Cartridges
Equipments
Flasks
Liquid ampoules
PE and PP single dose ampoules
Powders
DONNÉES TECHNIQUES
- Linear design and intermittent automatic movement
- Format of loads : diam. from 26 to 78 mm, height up to 150 or 300 mm, neck from 20 to 32 mm
- Output from 9,000 to 36,000 uph
- Manual loading or in-line conveyor
- Handling of the bottles by universal gripper, at the neck without any size part
- Washing with purified or recycled water, final rinsing with PPI water, blowing with sterile filtered air.
- Removable coaxial needles without accessories
- Technical area separate from the washing area
- Direct connection to the depyrogenation tunnel with synchronization
- Recycled water flow rate: 3 900 L/h
- PPI water flow: 300 to 1300 L/h, 3.5 bar
- Filtration through washing fluid at 2.5 µm then 0.45 µm, sterile compressed air 0.2 µm
- Options: Pre-washing cycle with ultra-sound bath, Sterilization of washing unit with steam, Drying cycle with hot air, Siliconization cycle, Production data recording soft-ware in accordance with 21CFR part 11, IQ / OQ Validation package and documentary traceability