Feedback on compliance and parameters recommended and requested by the European Pharmacopoeia & the US Pharmacopoeia for pure water in the pharmaceutical industry
According to the following 4 points:
- conductivity
- the C.O.T.
- bacteriology
- endotoxins
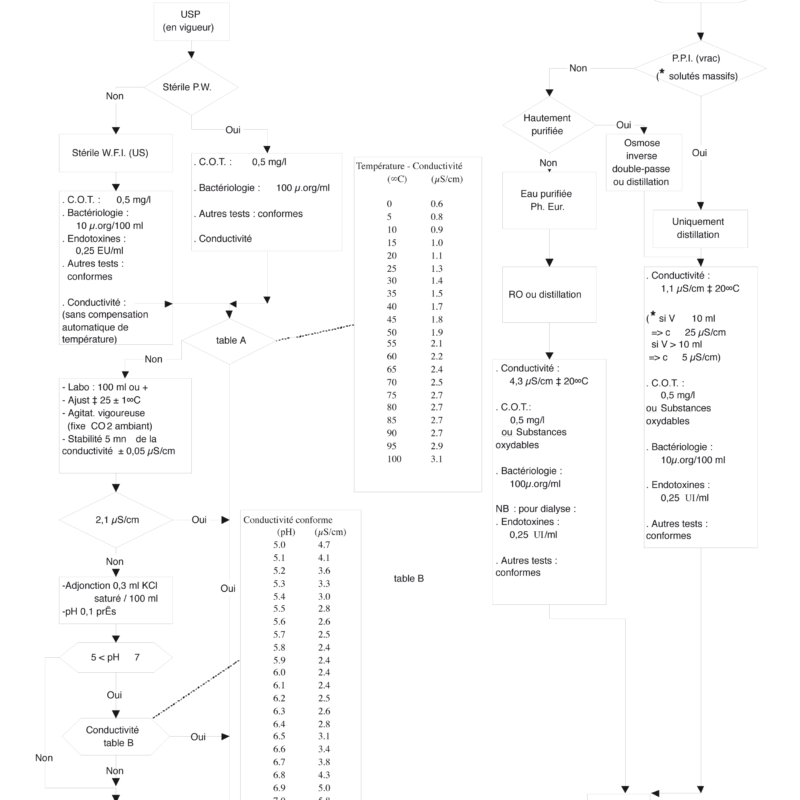
PURIFIED WATER
Water intended for the preparation of medicines other than those which must be sterile and free of pyrogens, unless justified and authorized exception.
HIGHLY PURIFIED WATER
Water intended for use in the preparation of medicinal products where water of high biological quality is required, except where the use of Water for Injections is required.
WATER PPI
Water intended either for the preparation of drugs for parenteral administration using an aqueous vehicle (water for bulk injection preparations), or for the dissolution or dilution of substances or. preparations for parenteral administration (sterilized water for injection preparations).
Find the excellent report on WHO Good Manufacturing Practices on water for pharmaceutical use.
Report written around: general principles applicable to pharmaceutical water systems, water quality specifications, application of specific water qualities to pharmaceutical processes and forms, water purification systems, systems storage and distribution of water, operational remarks and inspection of water systems.
*This document is a revision of the WHO Good Manufacturing Practices for Water for Pharmaceutical Use, previously published in the WHO Technical Report Series, No. 929, Annex 3, 2005

