HEAT TRANSFER ASPECT
Diagram of the heat transfer aspect of water vapor.
Find below the diagram which relates enthalpy and temperature (from -15°C to 200°C) under constant pressure (2 bar absolute and 1 bar relative) of the different states of water (solid, liquid, gas ). This diagram will also show you and easily highlight the difference between wet saturated steam, dry saturated steam and superheated steam.
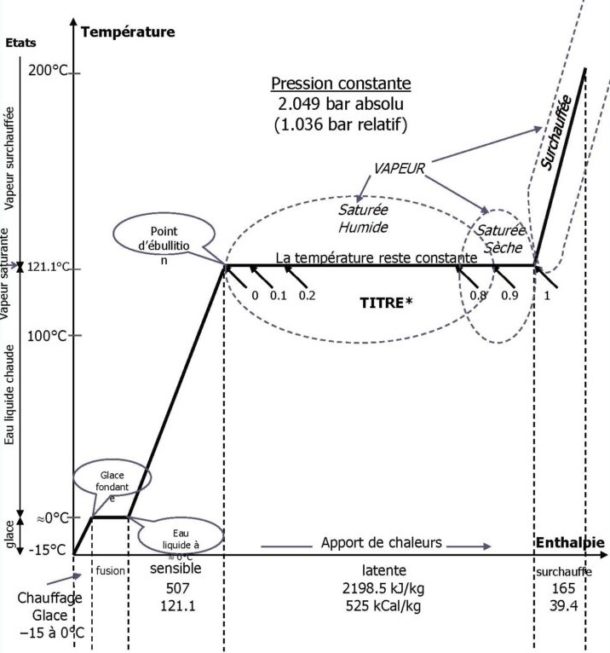
*Definition of the TITLE of saturated steam: in the two-phase gas mixture, it is the ratio of the masses of the vaporized fraction to the total mass of the steam and water mixture.
Several phases are observable for the states of water: a heating phase, a melting phase, a sensitive phase, a latent phase and a final superheated phase depending on the heat input. Note that each phase described above corresponds to a more or less defined state of the water. Specific points are also to be found: the points of melting ice, the beginning of liquid water and, most importantly, the boiling point.
STERIGENE, and its partner STILMAS, offer complete systems for producing pure fluids and in particular water vapor. The main feature is that it complies with current pharmaceutical industry standards. We can study with you, depending on your needs and constraints, the creation and complete installation of a water loop with integrated storage and steam production.
If you would like more information on these systems, do not hesitate to see our process equipment for producing pure fluids on our pages dedicated to industries but also to laboratories: PROCESS EQUIPMENT
If you would like more information on pure fluids, do not hesitate to refer to our numerous training courses which cover this subject by clicking here: TRAINING

